In recent years, MXene, a graphene-like structure obtained by MAX phase treatment, has attracted extensive research attention, and many partners are curious about this material. Today, Xiaobian will take you to understand the popular 2D material MXene.
1
What is MXene?
MXene is a graphene-like structure obtained by
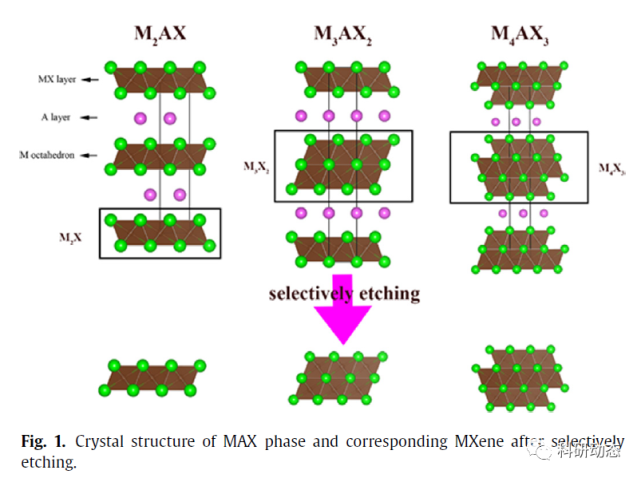
Max Phase treatment. The specific molecular formula for the MAX phase is Mn + 1AXn(n = 1, 2 or 3), where M refers to the transition metals of the previous groups, A refers to the main group elements, and X refers to the C and/or N elements.
Because M-X has A strong bond energy and A has a more active chemical activity, A can be removed from the MAX phase by etching to obtain a graphene-like 2D structure - MXene.
Figure 1. Crystal structure of the MAX phase and the corresponding etched MXene
Since the first report of MXene (Ti3C2Tx, where T stands for terminal of the surface, including OH, O or F) in 2011, a wide variety of MXene materials have been prepared in laboratories. Khazaei et al. proposed that the ground state of many MXene materials (Cr2CT2 or Cr2NO2) is ferromagnetic, and that the Seebeck parameters of semiconductor MXene are super-high at low temperatures. Zhang et al. first proposed that MXene (Ti2CO2) monolayers have two orders of magnitude higher hole mobility and lower electron mobility, and later confirmed high carrier mobility in experiments. Due to its unique properties, MXene has been widely used in catalysts, ion screening, photothermal conversion, field effect transistors, topological insulators and hydrogen evolution reactions.
2
How is MXene prepared?
As described above, Ti3C2Tx has been prepared since Naguib et al for the first time by selective etching with hydrofluoric acid (HF) at room temperature (RT). More and more researchers are working to find new ways to make more MXene. Naguib et al. first proposed that after removing the A (Al) layer, the MX (
Ti3C2) layer can be separated from the MAX (
Ti3AlC2) phase, and then through ultrasonic treatment, a new 2D Ti3C2 phase can be obtained. Then the effects of etching time, temperature, particle size and source of Ti3AlC2 on the preparation of 2D Ti3C2 by HF method were systematically studied. In addition, the strength of the A bond also determines the etching conditions. Selecting suitable etching conditions is the key to obtain high yield and purity.
Subsequently, in experiments with the same etching agent HF, more and more MXene was successfully obtained, Including Ti2CTx, TiNbCTx, Ti3CNxTx, Ta4C3Tx, Nb2CTx, V2CTx, Nb4C3Tx, Mo2CTx, (Nb0.8Ti0.2)4C3Tx, (Nb0.8Zr0.2)4C3Tx, Zr3C2Tx and Hf3C2Tx, of which Mo2C is the first MXene prepared by
Mo2Ga2C phase instead of MAX phase. In addition, Zr3C2 is an MXene prepared from Zr3Al3C5, which is a typical layered ternary and quaternary
Transition Metal carbide with a general formula for MnAl3Cn+2 and Mn[Al(Si)]4Cn+3, where M stands for Zr or Hf and n equals 1-3. A new MXene, Hf3C2Yx, was obtained by selective etching Hf3[Al(Si)]4C6. This result opens the door to the preparation of novel MXene from more diverse precursors. In addition to the typical terpolymer MXene, Anasori et al. calculated and predicted the ordered double M2D carbides M'M 'Xene by density functional theory (DFT), and prepared Mo2TiC2Tx, Mo2Ti2C3Tx and Cr2TiCxTx by using HF solution as etching agent.



